Illustrate the addition reactions of alkenes and teach the preparation and purification of an organic liquid
A demonstration of the hydration of alkenes. Students react hex-1-ene, CH3CH2CH2CH2CH=CH2, with concentrated sulfuric acid, followed by water, to make hexan-2-ol, CH3CH2CH2CH2CH(OH)CH3. The hexan-2-ol is then separated and distilled as a teacher demonstration and the product is tested.
Many of the procedures in this experiment are demonstrated in our video Preparation of an organic liquid.
Lesson organisation
The student experiment and separation (steps 1–4 of the demonstration) will fit well into a 45 minute lesson. The hexan-2-ol can then be allowed to dry overnight and the distillation and tests carried out in another 45 minute lesson.
Equipment
Apparatus
Each group of students will need:
- Goggles
- Disposable nitrile gloves
- Beaker (250 cm3)
- Measuring cylinder (5 cm3 or 10 cm3), 2
- Boiling tubes, 2
- Glass stirring rod
- Mineral wool
For the separation and distillation the teacher will need:
- Eye protection
- Beaker (250 cm3)
- Separating funnel (250 cm3)
- Conical flask (250 cm3)
- Filter funnel
- Filter paper
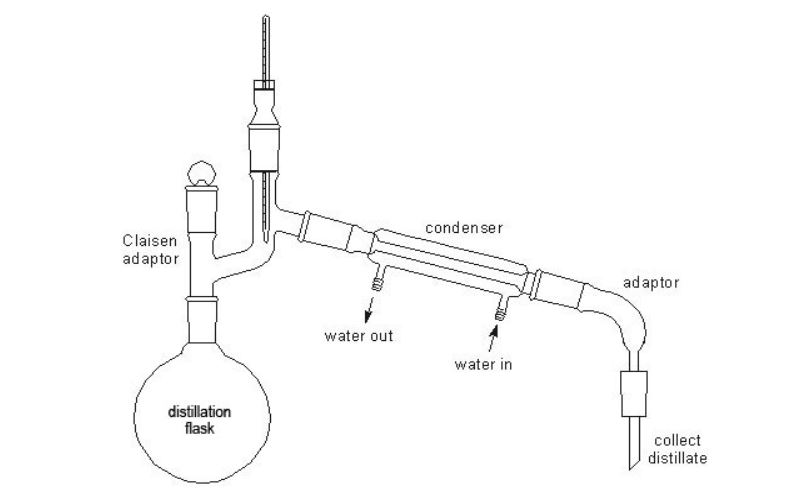
- Distillation apparatus, with thermometer (0–200°C or 0–250°C) (see diagram)
- Test-tube rack
- Test-tubes, with corks, 8
- Forceps
- Scalpel
- Distilled or deionised water, 10 cm3
Chemicals
Each group of students will need:
- Ice, a few lumps
- Hex-1-ene (IRRITANT, HIGHLY FLAMMABLE), 5 cm3
- Sulfuric acid, 75% (CORROSIVE), 5 cm3
For the separation and distillation the teacher will need:
- Anhydrous sodium carbonate (IRRITANT), 25 g
- Bromine water, 0.01 M (HARMFUL at this concentration), about 1 cm3
- Acidified potassium manganate(VII) solution, about 0.001 M, about 1 cm3
- Sodium (HIGHLY FLAMMABLE, CORROSIVE), a small piece no bigger than 2 mm
Health, safety and technical notes
- Read our standard health & safety guidance
- Wear goggles throughout and use protective gloves as appropriate (refer to CLEAPPS guidance, see p18 for recommended use of gloves).
- Hex-1-ene (IRRITANT, HIGHLY FLAMMABLE) – see CLEAPSS Hazcard HC045c.
- Sulfuric acid (CORROSIVE) – see CLEAPSS Hazcard HC098a and CLEAPSS Recipe Book RB098.
- Anhydrous sodium carbonate (IRRITANT) – see CLEAPSS Hazcard HC095A.
- Bromine water (HARMFUL at concentration used) – see CLEAPSS Hazcard HC015b and CLEAPSS Recipe Book RB017.
- Potassium manganate(VII) solution – see CLEAPSS Hazcard HC081 and CLEAPSS Recipe Book RB073.
- Sodium (HIGHLY FLAMMABLE, CORROSIVE) – see CLEAPSS Hazcard HC088.
- Hex-1-ene is a slightly unusual reagent which may have to be ordered specially. A fume cupboard is suggested for transferring and dispensing the liquid, and all should avoid inhaling the vapour. The laboratory should be well ventilated, and mineral-wool plugs used to minimise the escape of vapour.
- In order for schools to prepare their own 75% sulfuric acid solutions, Technicians should add 60 g of ice to a large beaker and put it on a magnetic stirrer with stirring flea. Wearing goggles or a face shield and corrosion resistant gloves, add 95% (‘concentrated’) sulfuric acid a little at a time 5 cm3 – not more than 50 cm3 to begin with. Then cautiously add further 50 cm3 aliquots until a volume of 250 cm3 solution is reached – this should be bottled for the experiment and labelled (CORROSIVE).
- Bromine water can also be purchased as the diluted solution.
- The potassium manganate(VII) is best purchased as a 0.02 M solution as making it up from the solid is difficult. For this purpose it should be diluted to a pale pink colour with dilute (1 M) sulfuric acid (IRRITANT).
- Using forceps, remove a piece of sodium from the oil, and place it on a tile. Ensure that conditions are dry. Using a scalpel or sharp knife, cut a small piece of sodium no larger than 2 x 2 x 2 mm. Place the small piece in a separate bottle of oil, or use immediately. Return the larger piece to its bottle.
Procedure
- Place a few lumps of ice and some water in a 250 cm3 beaker, to make an ice bath.
- Measure out 5 cm3 of hex-1-ene using a measuring cylinder and pour it into a boiling tube, add a mineral wool bung.
- Cool the hex-1-ene in the ice bath for about three minutes.
- Measure out 5 cm3 of 75% sulfuric acid into another measuring cylinder.
- Slowly add the acid to the hex-1-ene, keeping the tube in the ice bath and stirring the mixture with a glass rod.
- When all the acid has been added, continue to stir the mixture until it becomes homogeneous (just one layer is visible). This will take about 5 minutes. Replace the mineral wool bung.
- Allow the mixture to stand in the ice bath for another 5 minutes, then carefully add an equal volume of cold water, using another boiling tube. The mixture should separate into two layers. The top layer is impure hexan-2-ol, the lower layer contains mainly sulfuric acid.
Separation and distillation of the product
- Ask students to tip the contents of their boiling tubes into a beaker. Then pour the contents into a separating funnel, stopper, shake and allow the contents to settle.
- Remove the stopper and run off the lower layer (containing sulfuric acid) into the beaker and discard it by washing it carefully down the sink with plenty of water.
- Add 10 cm3 distilled water to the separating funnel, stopper and shake. This washes the hexan-2-ol. Remove the stopper and run off the bottom aqueous layer and discard it.
- Place 25 g of anhydrous sodium carbonate in a 250 cm3 conical flask. Run the hexan-2-ol out of the separating funnel into the flask. Swirl the flask frequently for twenty minutes to dry the hexan-2-ol.
- Set up a distillation apparatus (see diagram). Decant as much as possible of the hexan-2-ol from the conical flask into the distilling flask and then filter the rest into the distilling flask.
6. Distil, collecting the fraction that boils between 130o and 160oC. This is hexan-2-ol. Note that hex-1-ene boils at a much lower temperature, 63°C.
7. Using a few drops of hex-1-ene and hexan-2-ol for each test, compare them by:
- Shaking (in a corked test-tube) with about 1 cm3 bromine water.
- Shaking (in a corked test-tube) with about 1 cm3 acidified potassium manganate(VII) solution.
- In a clean dry test tube, adding a small piece of sodium metal.
- Placing a few drops on a crucible lid and igniting with a lit splint.
Teaching notes
The overall preparation reaction is:
C6H12 + H2O → C6H13OH
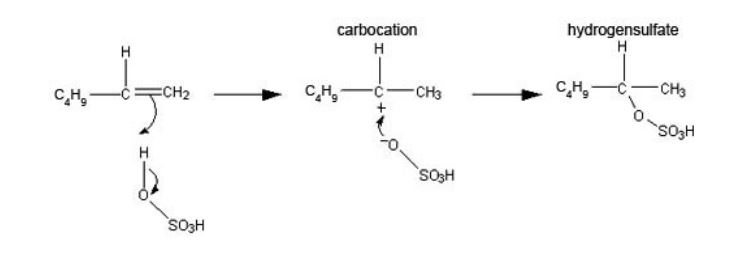
The mechanism is shown by:
The secondary carbocation is formed rather than the primary one where the positive charge is on the end carbon. This is because of stabilisation by inductive effects from the two carbon atoms and is an example of Markovnikov’s rule.
The alkyl hydrogensulfate reacts with water to give the alcohol and regenerates sulfuric acid, but of course it is the secondary alcohol hexan-2-ol, CH3CH2CH2CH2CH(OH)CH3, that is formed, rather than the primary hexan-1-ol.
Hexan-2-ol has hydrogen bonding between its molecules giving it a much higher boiling point than hex-1-ene, which just has van der Waals’ forces.
The test reactions are:
Bromine water
C6H12 + Br2 → C6H12Br2
1,2-dibromohexane is formed. The brown colour of the bromine is decolorised; there is no reaction with hexan-2-ol
Potassium manganate(VII)
Potassium manganate(VII) saturates the double bond in hexene by oxidation – the purple colour is decolorised; there is no reaction with hexan-2-ol in the cold.
Sodium
2C6H13OH + 2Na → 2C6H13ONa + H2
Fizzing should be seen; there is no reaction with hexene.
Combustion
The hexene should burn with a more smoky flame than the alcohol because it is unsaturated.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet