Use this class practical or demonstration to produce ethene gas as an example of an unsaturated hydrocarbon
In this experiment, students observe how to produce ethene gas by dehydrating ethanol vapour over a heated catalyst. After collecting the gas over water, students can test it for the typical properties of an unsaturated hydrocarbon.
The experiment can be performed as a demonstration or as a class experiment according to circumstances. The main risk to be considered in making the choice is the reliability of the student’s involved handling very hot glassware and manipulating the apparatus for safe gas collection over water, while avoiding suck-back of cold water into the hot tube.
A demonstration will take about 10–15 minutes, with a few more minutes for testing the gases. A class experiment takes longer, probably about 30–40 minutes.
Alternatively the teacher could demonstrate the dehydration but get the students to perform the test tube tests for the product.
Equipment
Apparatus
- Eye protection
- Safety screens (for a demonstration)
- Boiling tube (see note 8 below)
- Rubber bung, 1 hole, to fit boiling tube
- Delivery tube (see diagram below)
- Bunsen valve (see note 9)
- Test tubes, x6
- Corks or bungs, to fit test tubes, x6
- Dropping pipette
- Bunsen burner
- Heat resistant mat
- Test tube rack
- Glass or plastic trough, small (see note 10)
- Wooden splint
- Retort stand, boss and clamp
Chemicals
- Ethanol (IDA, industrial denatured alcohol) (HIGHLY FLAMMABLE, HARMFUL), 2–3 cm3 (provided in small bottles)
- Mineral wool, sufficient for a loosely-packed wad to absorb the liquid at the bottom of the test tube
- Pumice stone or porous pot (unglazed), a supply of small fragments (about pea-sized)
- Bromine water, 0.02 M (HARMFUL), about 5 cm3, diluted to pale orange-brown
- Acidified potassium manganate(VII) (potassium permanganate) solution, about 0.01 M, about 5 cm3 (see note 6 below)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- For a demonstration, the class and teacher should be protected by safety screens in case of unexpected suck-back causing the hot tube to shatter on a demonstration bench.
- Ethanol (IDA, Industrial Denatured Alcohol) (HIGHLY FLAMMABLE, HARMFUL) - see CLEAPSS Hazcard HC040a.
- Bromine water (HARMFUL) - see CLEAPSS Hazcard HC015b.
- Potassium manganate(VII) (potassium permanganate) solution, about 0.01 M - see CLEAPSS Hazcard HC081 and CLEAPSS Recipe Book. The potassium permanganate(VII) solution is made by dissolving solid potassium permanganate(VII) in 0.1 M sulfuric acid. 1 dm3 of stock solution is made by dissolving 1.6 g of potassium permanganate(VII) crystals in 1 dm3 of 0.1 M sulfuric acid.
- Dilute sulfuric acid, 0.1 M - see CLEAPSS Hazcard HC098a and CLEAPSS Recipe Book.
- A large, 150 mm x 25 mm, hard glass (borosilicate) test tube.
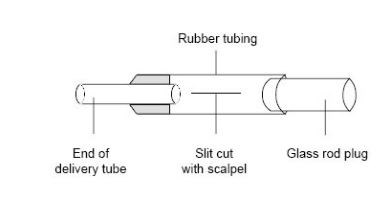
- The purpose of the Bunsen valve is to prevent suck-back of water into the hot tube when heating is stopped and the gas inside the apparatus contracts on cooling. The Bunsen valve is constructed from a short length (about 3 cm) of clean, unused, soft rubber tubing, with one end stoppered with a short length of glass rod. A 1 cm long slit is carefully cut into the middle of the rubber tubing with a scalpel. The resulting assembly is fitted onto the lower end of the delivery tube. Note that the effectiveness of the home-made valve is variable, and for class use a supply of spares should be provided.
- The trough needs to be small enough to match the scale of gas collection – the large traditional trough which used to be used with gas jars is not appropriate. For demonstration or class use square, clear, plastic sandwich boxes make excellent troughs.
Procedure
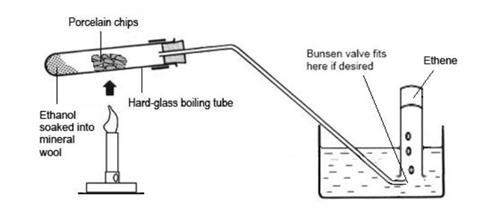
- Assemble the apparatus within easy reach of a Bunsen flame.
- Half fill the trough with water and fill the test tubes with water, leaving them submerged in the trough. Bungs for the test tubes can be placed upside-down in the trough, so that the tubes filled with gas can just be pressed onto them before they are removed to a rack.
- Place a wad of mineral wool in the bottom of the boiling tube so that it fills the bottom to a depth of about 1 cm without packing too firmly. Using a dropping pipette, carefully drop about 2 cm3 of ethanol into the mineral wool so that it soaks in. It should be possible to invert the tube without any significant amount of liquid draining out.
- Clamp the boiling tube at the neck end so that the mouth is tilted slightly upwards. Fill most of the tube with pieces of pumice stone or broken porous pot. This will ensure maximum contact time between the ethanol vapour and the hot catalyst. Fit the delivery tube so that it dips into the water in the trough. Fit a Bunsen valve, if desired.
- Heat the pumice stone or porous pot strongly with the tip of medium Bunsen flame for several seconds until thoroughly hot. Avoid heating the tube too close to the rubber stopper. Then flick the flame quickly onto the mineral wool for a few seconds to vaporise some of the ethanol. Then return the flame to the catalyst.
- When a steady stream of gas bubbles is established, collect four to six tubes full of gas by holding them over the Bunsen valve. It is better to have an assistant to manipulate the gas collection tubes, changing and sealing them as they are filled. Take care not to lift the water-filled tubes out of the water when moving them, to avoid letting air into them. Seal the full tubes by pressing them down on the bungs, then place them in a rack.
- Continue with the Bunsen burner heating the mineral wool for about one second out of every ten and the pumice stone for the other nine. To avoid the risk of suck-back, do not remove the Bunsen burner flame from the heated tube while the gas is being collected. If suck-back becomes unavoidable, quickly remove the delivery tube from the water by lifting the whole apparatus using the clamp stand.
- When six tubes of gas have been collected, or gas production ceases, remove the delivery tube from the water by lifting the clamp stand, and then stop heating. Keep the Bunsen flame away from the end of the delivery tube.
- Test the tubes of collected gas as follows:
- Pass the first two tubes (mainly air anyway) round the class so that the students can cautiously smell the gas.
- Uncork the last tube collected, and hold a lighted spill in its mouth to ignite the gas. It will burn with a yellow flame.
- To another test tube of gas, add about 1 cm depth of bromine water. Re-seal it and shake well. If the pale orange bromine water is decolourised, the gas contains an unsaturated hydrocarbon (a bromoalkanol).
- Add about 1 cm depth of acidified potassium permanganate(VII) solution to another test tube of gas. Re-seal and shake well. If the purple solution is decolorised, possibly leaving a brown coloration of manganese dioxide, the gas contains an unsaturated hydrocarbon (a diol).
- The results of the last two tests are characteristic of unsaturated hydrocarbons, whose molecules contain carbon-carbon double bonds.
Teaching notes
This experiment would form part of the teaching and learning sequence for the chemistry of hydrocarbons and alcohols. The teacher will need to decide:
- How much the students should know about hydrocarbons before this lesson
- If they are familiar with hydrocarbons, whether they should be familiar with the potassium manganate(VII) and bromine water tests for a carbon-carbon double bond before the lesson, or should be introduced to these tests by means of this experiment
For the dehydration of ethanol, the only likely product is ethene. Ball and stick molecular models will be useful for modelling both the dehydration and the tests.
The ethanol dehydration is represented by:
CH3 CH2 OH(g) → CH2 =CH2 (g) + H2 O(g)
The experiment can be linked to the industrial applications of such reactions; see the suggestions for further reading below.
Bromine in non-aqueous solution adds across the double bond of an alkene, to give for example 1,2-dibromoethane, CH2 Br–CH2 Br (a suspected carcinogen). However, in aqueous solution the main product is a bromoalkanol, such as 2-bromoethanol, CH2 Br–CH2 OH .
Acidified potassium permanganate(VII) acts as an oxidising agent on the double bond to produce diols such as ethan-1,2-diol, CH2 OH–CH2 OH. Under harsher conditions the diols cleave to form two carbonyl compounds (ketones or carboxylic acids).
Industrially some ethanol is made by the reverse reaction, the addition of water to ethene. However, a really important dehydration of an alcohol is the dehydration of 1-phenylethanol to produce phenylethene, better known as styrene, the monomer for polystyrene.
Further reading
Try the resources below to review the dehydration of ethanol and other alcohols to alkenes by various methods:
- A discussion of the dehydration of alcohols using aluminium oxide as a catalyst (Chemguide)
- A discussion of the mechanism of the dehydration of ethanol, for post-16 students and teachers (Chemguide)
- An overview of the manufacture of ethanol from ethene (Greener Industry)
- An explanation of the process and conditions required for the manufacture of ethanol (Chemguide)
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry

















No comments yet