Use this class practical to practise locating the end-point of a titration by measuring the temperature change
In this experiment, students titrate sodium hydroxide solution with hydrochloric acid. By measuring the temperature change each time a portion of acid is added, students can determine the end-point of the titration, indicated by the highest temperature. They then use this information to calculate the concentration of the hydrochloric acid.
The practical takes about one hour, and is best carried out individually or in pairs.
Equipment
Apparatus
- Eye protection (goggles)
- Thermometer, 0–100 °C (see note 5 below)
- Two insulated (polystyrene) cups
- Beaker, 250 cm3
- Burette, 50 cm3
- Burette stand
- Clamp and stand (optional)
- Cork, one-holed, to fit thermometer (optional)
- Pipette, 20 cm3 or 25 cm3
- Pipette safety filler
Chemicals
- Hydrochloric acid, 2.00 M (IRRITANT), about 75 cm3
- Sodium hydroxide solution, 1.50 M (CORROSIVE), about 30 cm3
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Hydrochloric acid, HCl(aq), (IRRITANT at concentration used) – see CLEAPSS Hazcard HC047a and CLEAPSS Recipe Book RB043. This concentration is necessary to achieve a reasonable change in temperature. The concentration of the hydrochloric acid should not be indicated on bottle available to the students.
- Sodium hydroxide solution, NaOH(aq), (CORROSIVE at concentration used) – see CLEAPSS Hazcard HC091a and CLEAPSS Recipe Book RB085. This concentration is necessary to achieve a reasonable change in temperature. The concentration of the sodium hydroxide should be indicated on bottle available to the students.
- Instead of using the thermometer to stir the titration mixture, it could be clamped in position in a cork, as shown in the diagram, and the mixture swirled after each addition of acid. Alternatively, a temperature sensor attached to a computer can be used in place of a thermometer. Data logging software could then be used to provide a detailed plot of the readings.
Procedure
- Stand an insulated cup in a beaker for support.
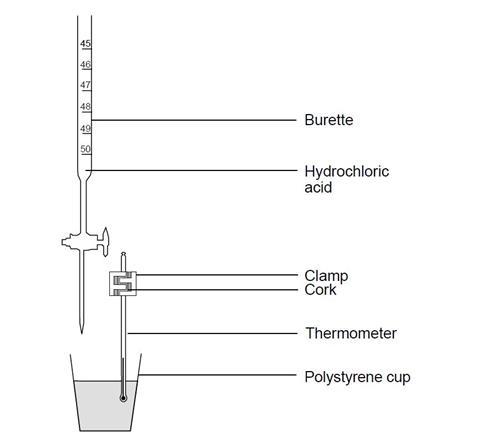
- Using a pipette and safety filler, transfer 20 cm3 (or 25 cm3) of the sodium hydroxide solution into the cup, and measure the steady temperature.
- Using the burette, add a small portion (3–5 cm3) of dilute hydrochloric acid to the solution in the cup, noting down the actual volume reading. Stir by swirling the cup and measure the highest temperature reached.
- Immediately add a second small portion of the dilute hydrochloric acid, stir, and again measure the highest temperature and note down the volume reading.
- Continue in this way until there are enough readings to decide the maximum temperature reached during this experiment. You will need to add at least 30 cm3 of the acid.
- Plot a graph of temperature against the volume of acid added, and use extrapolation of the two sections of the graph to deduce the maximum temperature reached without heat loss.
- Use your results to calculate the concentration of the hydrochloric acid.
Teaching notes
The main concern in this experiment is the heat loss. If possible, a lid should be used. More reliable results can be achieved using two polystyrene cups (one inside the other).
With abler or older students, it is possible to discuss the extrapolation of the cooling curve to estimate the maximum temperature reached without heat loss. Creative Chemistry provide a resource on thermometric titration which includes an example of a typical plot of temperature vs volume of acid for this experiment, as well as the use of extrapolation to determine the maximum temperature change.
To reinforce the theory involved here, an indicator could also be used to show that the end-point really did occur at the highest temperature.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry

















No comments yet