Compare the thermal stabilities of carbonates of reactive metals, like sodium and potassium, and the carbonates of less reactive metals, including lead and copper
Metal carbonates decompose when heated. Some carbonates are more reactive than others. The aim of this experiment is to compare the reactivity of some different metal carbonates.
This experiment can be carried out as a class exercise, individually or in pairs. Because of the toxicity of lead compounds, it may be best to leave lead carbonate out with less reliable classes.
The experiment should take about 40–45 minutes.
Equipment
Apparatus
- Eye protection
- Test tubes x2 (per carbonate)
- Delivery tube (right-angled)
- Spatula
- Bunsen burner
- Clamp and stand
Chemicals
- Limewater (calcium hydroxide solution), 10 cm3 per carbonate
- About 2 g each of following solids:
- Copper carbonate (HARMFUL)
- Lead carbonate (TOXIC, DANGEROUS FOR THE ENVIRONMENT)
- Potassium carbonate (IRRITANT)
- Sodium carbonate, anhydrous (IRRITANT)
- Zinc carbonate
Health, safety and technical notes
- Read our standard health and safety guidance
- Wear eye protection. It is important not to inhale dust of lead carbonate or the oxide formed. Wash hands after using lead compounds.
- Limewater (calcium hydroxide solution), Ca(OH)2(aq), (treat as IRRITANT) – see CLEAPSS Hazcard HC018 and Recipe Book RB020.
- Copper carbonate, CuCO3.Cu(OH)2(s), (HARMFUL) – see CLEAPSS Hazcard HC026.
- Lead carbonate, PbCO3(s), (TOXIC, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC056.
- Potassium carbonate, K2CO3(s), (IRRITANT) – see CLEAPSS Hazcard HC095a.
- Sodium carbonate, anhydrous, Na2CO3(s), (IRRITANT) – see CLEAPSS Hazcard HC095a.
- Zinc carbonate, ZnCO3(s) – see CLEAPSS Hazcard HC108b.
Procedure
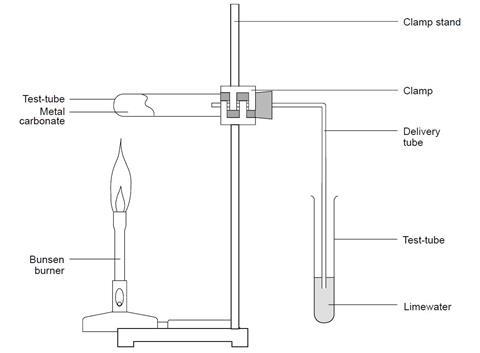
- Put a large spatula measure of the carbonate to be tested in a test tube.
- Fit a delivery tube and then clamp the test tube so that the delivery tube dips into a second test tube containing 2–3 cm3 limewater.
- Heat the solid gently at first, then more strongly.
- Lift the delivery tube from the limewater before, or as soon as, the heating is stopped. This is to avoid suck-back.
- Write down all observations. Notice what happens to the limewater and how long it takes to turn milky (if at all). Notice whether any melting occurs in the heated test tube and any colour changes taking place. Write your results in tabular form.
- Repeat the experiment with the other metal carbonates supplied, and in each case write down your observations.
- Wash your hands thoroughly at the end of these experiments, since some of the metal carbonates are toxic.
Teaching notes
It is important to emphasize how suck-back can be avoided before the students begin, otherwise there are bound to be mishaps.
It is also important to ensure that students wash their hands after using lead carbonate, and to ensure that dust is not raised when this solid is being used.
The relative ease with which the carbonates of some of the less reactive metals are decomposed has been used in the extraction of these metals from ores that contain the metal as a carbonate, for example zinc carbonate (calamine).
|
Carbonate |
Colour before heating |
Colour after heating |
Gas evolved |
Ease of decomposition |
|
Potassium carbonate |
White |
White |
None |
Very difficult |
|
Sodium carbonate |
White |
White |
None |
Very difficult |
|
Zinc carbonate |
White |
Yellow when hot, white when cool |
Carbon dioxide |
Fairly easy |
|
Lead carbonate |
White |
Yellow |
Carbon dioxide |
Fairly easy |
|
Copper carbonate |
Green |
Black |
Carbon dioxide |
Easy |
Students should find that sodium and potassium carbonates give no carbon dioxide or any other sign that decomposition has taken place, even after prolonged heating.
Those metal carbonates which do decompose leave a residue of the metal oxide and evolve carbon dioxide in the process, eg:
ZnCO3(s) → ZnO(s) + CO2(g)
At an elementary level, the relative thermal stability of the carbonates of the metals cannot easily be explained in terms of simple ideas of bonding in these compounds. A simple relationship between the reactivity of the metal and the stability of its compounds, such as the carbonate here, will have to suffice.
With abler and older students it may be appropriate to refer to the polarization (distortion) of the electron cloud of the carbonate ion by the metal ion, and that this is bound to be more pronounced when the metal ion is doubly, rather than singly charged, and small. Polarization eventually leads to abstraction of oxygen from the carbonate ion, producing the oxide ion and a carbon dioxide molecule. The greater the polarization, the lower the temperature needed to decompose the carbonate.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry

















No comments yet