This class experiment or demonstration explores some of the chemical properties of halogens, comparing the colours of three halogens in aqueous solution and in a non-polar solvent, and observing their bleaching properties and displacement reactions
Halogens react to a small extent with water, forming acidic solutions with bleaching properties. They also undergo redox reactions with metal halides in solution, displacing less reactive halogens from their compounds. These displacement reactions are used to establish an order of reactivity down Group 17 of the periodic table.
This series of simple experiments illustrates some of the chemical properties of the halogens following an introduction to the physical properties of the Group 17 elements. It can be done as a demonstration or as a class experiment.
Investigating the solubility of the halogens in a non-polar solvent can be left out, or only shown as a demonstration.
If the activity is done as a demonstration it should take around 15 minutes. If it is done as a class experiment you should allow 30 minutes.
Equipment
Apparatus
- Eye protection
- Test tube rack, to hold 10 test tubes
- Test tubes x10
- Cork or rubber bungs to fit, x4
- Plastic dropping pipettes x6
- White spotting tile
- White tile
- Glass rod
- Paper towel or tissue
Chemicals
- About 10 cm3 of each of the following halogen solutions in stoppered test tubes (see notes 1 and 2):
- Chlorine water, 0.1% (w/v) (HARMFUL)
- Bromine water, 0.1% (w/v) (HARMFUL)
- Iodine solution, 0.1 M
- Half a test tube of 0.1 M solutions of each of the following:
- Potassium chloride
- Potassium bromide
- Potassium iodide
- Universal indicator paper (about 2 cm strips), x3
Optional
- Cyclohexane (HIGHLY FLAMMABLE, HARMFUL, DANGEROUS FOR THE ENVIRONMENT) or other suitable non-polar solvent, about 10 cm3 (see note 1)
Notes on chemicals
- Each group of students should be supplied with stoppered test tubes containing about 10 cm3 of each of the aqueous solutions of the halogens and one of cyclohexane (optional).
- The halogen solutions can be diluted further to minimise the amount of chlorine or bromine fumes given off but should not be so dilute that their distinctive colours are not clearly visible in the test tubes (a white background may be needed for chlorine water).
- At the end of the experiments all mixtures and solutions should be returned to a suitable waste container in a fume cupboard for safe disposal.
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Take care to limit students’ exposure to chlorine and bromine water fumes. Some students with respiratory problems can show an allergic reaction to chlorine, the onset of which may be delayed.
- Chlorine water, Cl2 (aq) – see CLEAPSS Hazcard HC022b and CLEAPSS Recipe Book RB025. The solution itself is LOW HAZARD but chlorine gas (TOXIC, DANGEROUS FOR THE ENVIRONMENT) escapes, so a HARMFUL label would be sensible.
- Bromine water, Br2 (aq), (HARMFUL) – see CLEAPSS Hazcard HC015b and CLEAPSS Recipe Book RB017.
- Iodine solution, I2 (aq) – see CLEAPSS Hazcard HC054 and CLEAPSS Recipe Book RB050. Iodine solution is actually iodine dissolved in aqueous potassium iodide.
- Potassium chloride, KCl(aq), potassium bromide, KBr(aq) and potassium iodide, KI(aq) solutions are all LOW HAZARD – see CLEAPSS Hazcard HC047b and CLEAPSS Recipe Book RB068. The sodium salts can be used if the potassium salts are not available. The concentration of the potassium iodide solution should be adjusted so that it gives a light brown solution on addition of chlorine water. If the reagents are too concentrated, a black precipitate of iodine often results instead of a brown solution.
- Cyclohexane, C6 H12 (l), (HIGHLY FLAMMABLE, HARMFUL, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC045b.
Procedure
The halogens in water and a hydrocarbon solvent (optional)
- Pour about 2 cm3 of each of the aqueous halogen solutions into separate test tubes. Add equal volumes of hydrocarbon solvent to each tube, stopper the tube and, holding your thumb over the bung, shake the mixture by inverting the test tube a few times.
- Allow the two layers to settle. Observe and record the colour of each layer. It may be necessary to shake the test tubes again to transfer more of the halogen from the water to the hydrocarbon layer.
Acidic and bleaching properties of halogen solutions
- Place a piece of universal indicator paper on a white tile. Transfer a drop of chlorine water onto the paper using a glass rod. Observe and record the colour of the paper.
- Wipe the glass rod and the tile clean with a paper towel or tissue. Place a fresh piece of indicator paper on the tile and transfer a drop of bromine water onto it using the glass rod. Observe the colour of the paper.
- Repeat step 2, using the iodine solution.
Displacement reactions
- Using a plastic pipette put two drops of chlorine solution in each of three dimples in the spotting tile, as shown below. In the same way and using a clean plastic pipette for each solution, add bromine water and iodine solution to the spotting tile.
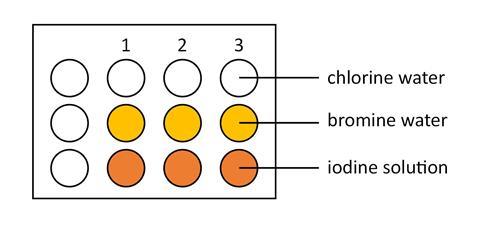
- Add two drops of potassium chloride solution to each of the three dimples in column 1 of the tile. Observe and record any colour changes that take place.
- Add two drops of potassium bromide solution to each of the three dimples in column 2 of the tile. Observe and record any colour changes that take place.
- Add two drops of potassium iodide solution to each of the three dimples in column 3 of the tile. Observe and record any colour changes that take place.
Optional
- For reactions in which bromine or iodine are suspected to have formed, the reaction could be repeated with 2 cm3 of each solution in a test tube, and hexane could then be added to confirm the presence of bromine or iodine.
Teaching notes
A results table similar to the one below could be used for the recording of results. It has been completed with expected observations.
| Colour after shaking with hydrocarbon solvent | Effect on indicator paper | Reaction with potassium chloride solution | Reaction with potassium bromide solution | Reaction with potassium iodide solution | |
|---|---|---|---|---|---|
| Chlorine water |
Aqueous layer: pale yellow-green to colourless Hydrocarbon layer: colourless to pale yellow-green |
Turns red then rapidly bleaches white |
No reaction |
The yellow-orange colour of bromine appears |
The brown colour of iodine appears |
| Bromine water |
Aqueous layer: yellow-orange to colourless Hydrocarbon layer: colourless to pale yellow-orange |
Turns red then slowly bleaches white |
No reaction |
No reaction |
The colour darkens from yellow-orange to brown |
| Iodine solution |
Aqueous layer: brown to colourless Hydrocarbon layer: colourless to purple |
The paper is stained brown |
No reaction |
No reaction |
No reaction |
The halogens are more soluble in the hydrocarbon and move to this top layer when shaken with a hydrocarbon solvent. For chlorine and bromine the colour does not change. You might need a white background to see the colour of the chlorine solution. However, for iodine there is a colour change, from brown in water to purple in the hydrocarbon layer.
Where no displacement reaction takes place between a halogen solution and a halide solution, it may be that some lightening in the colour of the solution is observed and this can be explained by the effect of dilution.
Take care to limit students’ exposure to chlorine and bromine water fumes. Some students with respiratory problems can show an allergic reaction to chlorine, the onset of which may be delayed.
Iodine is the least soluble of the halogens in water. It is more soluble in potassium iodide solution, so the ‘iodine solution’ here is actually iodine in potassium iodide solution.
Draw the students’ attention to the similarity between the colour of iodine vapour and its colour in a non-polar solvent. Polar water molecules interact with iodine molecules, altering the wavelengths of light they absorb.
All three halogens react with water to produce a strong acid (HX), and a weak acid (HOX), which has bleaching properties and is an oxidising agent.
X2(aq) + H2O(l) → HX(aq) + HOX(aq)
The extent of reaction decreases down Group 17. With iodine it is so small that the acidic and bleaching properties of the solution are not seen in this experiment.
In the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. For example:
Cl2(aq) + 2KI(aq) → I2(aq) + 2KCl(aq)
The order of reactivity is therefore: chlorine > bromine > iodine. A more advanced treatment identifies the halogens as oxidising agents, accepting an electron to form halide ions:
Cl2(aq) + 2I-(aq) → I2(aq) + 2Cl-(aq)
Contrary to belief among many students, the reaction has nothing to do with the reactivity of potassium ‘grabbing’ the chlorine. Potassium is only present here as very unreactive potassium ions (spectator ions) in solution.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet