Try this practical to investigate how much oxygen is used in rusting and calculate the percentage of oxygen in air
When iron rusts, water and oxygen in the air react with the iron to form iron oxide. In this experiment, students set up iron wool to rust in a test tube full of air, inverted in a beaker of water. As the iron wool reacts, rusts and removes the oxygen from the air, water is drawn up the tube. By observing the change in the volume of air in the tube, students can calculate the air’s oxygen concentration.
This experiment will need to be carried out over two lessons about a week apart. The practical work will probably take no more than 20 minutes in either lesson.
Equipment
Apparatus
- Test tube (see note 3 below)
- Beaker, 100 cm3
- Ruler
Chemicals
- Iron wool
Health, safety and technical notes
- Read our standard health and safety guidance.
- Iron wool, Fe(s) – see CLEAPSS Hazcard HC055A.
- The test tubes used in this experiment can get stained by the rust. They can be cleaned with a ‘Stain Devil’®.
Procedure
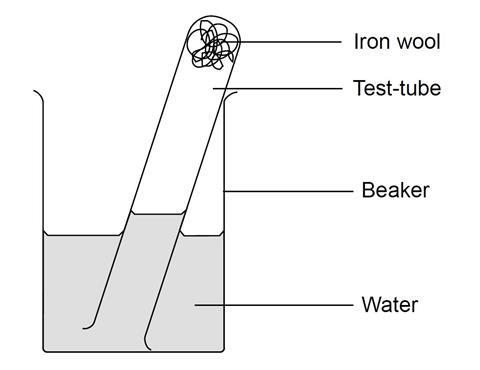
- Put about 3 cm depth of iron wool into the test tube and wet it with water. Tip away excess water.
- Put about 20 cm3 water into the beaker. Invert the test tube and place it in the beaker of water (see diagram). Measure the length of the column of air with the ruler.
- Leave for at least a week.
- Measure the new length of the column of air, taking care not to lift the test tube out of the water.
Teaching notes
Students need to understand that rusting is an oxidation reaction of iron with oxygen.
iron + oxygen → iron oxide
This is not the full story, however, as the formation of rust is a complex process. There are many websites searchable by Google which provide more detail as needed.
From their two measurements for the length of the column of air – before and after rusting takes place – students should be able to calculate the percentage of the air which has been removed by the rusting reaction. This should be about 20% which is approximately the percentage of oxygen in the air.
You could ask students how they could show that the reaction is complete – they may suggest leaving it for another week or so to see if any further air is used up. The iron is present in excess in this experiment, so it will not all rust. No more air will be consumed beyond 20% (assuming the equipment is sealed correctly) as all the oxygen has been used up.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry

















No comments yet