Use this demonstration to illustrate an exothermic redox reaction by heating iron wool in the presence of chlorine gas and the vapours of bromine and iodine
In this teacher demonstration, students observe what happens when iron wool is heated and chlorine gas and the vapours of bromine and iodine are passed over it. Students can watch the iron wool begin to glow as the expected exothermic redox reactions take place, forming iron(III) halides (FeX3) as coloured solids.
The vigour of the reactions corresponds to the order chlorine > bromine > iodine, showing the trend of decreasing reactivity of the elements down Group 17.
These experiments must be carried out in a fume cupboard as both the reactants and products are hazardous. Teachers attempting this demonstration for the first time are strongly advised to carry out a trial run before doing it in front of a class.
The time allowed should be at least 20 minutes, depending on the amount of discussion and testing of the products between each experiment.
In addition to using this demonstration to show the relative reactivity of the halogens, the reaction of chlorine or bromine with iron could be used on its own to show the reaction between a reactive non-metallic element and a metal.
Equipment
Apparatus
- Eye protection for teacher and students
- Protective gloves for teacher
- Access to a fume cupboard
- Apparatus to set up a chlorine generator
- Boiling tubes, x2
- Reduction tube (see note 3 below)
- Beakers, 100 cm3, x3
- Tweezers
- Teat pipette (preferably glass, with a narrow tip)
- Test tubes, small, x3
- Test tube rack
- Bunsen burner
- Heat resistant mat
- Bosses, clamps and stands
Chemicals
- Iron wool, 3 tufts about 1 g mass each (see note 4 below)
- Hexane (HIGHLY FLAMMABLE, HARMFUL), 100 cm3 (see note 5 below)
- Liquid bromine (VERY TOXIC, CORROSIVE), 0.5 cm3
- Sodium thiosulfate solution, 1 M, 500 cm3
- Iodine solid (HARMFUL), 0.5 g
- Silver nitrate solution, approximately 0.1 M, 10 cm3
- To generate chlorine (see notes 10, 11 and 12 below):
- Sodium chlorate(I) solution (sodium hypochlorite), 10–14% (w/v) (CORROSIVE), fresh
- Hydrochloric acid, 5 M (IRRITANT AT THIS DILUTION)
- Deionised or distilled water
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear suitable eye protection (goggles) throughout and work in a fume cupboard at each stage of this demonstration.
- The reduction tube should be fitted with a one-holed rubber stopper fitted with short length of glass tubing. Alternatively, an 8–10 cm length of wide-bore glass tubing with a stopper at each end fitted with a short length of glass tubing could be used. See diagram below.
- Iron wool, Fe(s) – see CLEAPSS Hazcard HC055A. The finest grade iron wool is best since it provides the maximum surface area. Iron wool is often sold with a thin layer of grease on its surface to stop it rusting. Working in a fume cupboard, the layer of grease can be removed by dipping the iron wool in hexane (or alternative) a few times. The solvent must be allowed to completely evaporate.
- Hexane, C6 H14 (l), (HIGHLY FLAMMABLE, HARMFUL, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC045a.
- Liquid bromine, Br2 (l), (VERY TOXIC, CORROSIVE, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC015a. See our standard guidance on handling liquid bromine and preparing bromine water. Wear suitable protective gloves when handling bromine and have at least 500 cm3 of 1M sodium thiosulfate readily available to treat any spillages.
- Sodium thiosulfate solution, Na2 S2 O3 (aq) – see CLEAPSS Hazcard HC095A.
- Iodine solid, I2 (s), (HARMFUL, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC054.
- Silver nitrate solution, AgNO3 (aq) – see CLEAPSS Hazcard HC087. Although LOW HAZARD at this concentration, the solution can still stain skin, clothes and some bench materials.
- For generating chlorine gas, Cl2 (g), (TOXIC, DANGEROUS FOR THE ENVIRONMENT), see CLEAPSS Hazcard HC022a and CLEAPSS Recipe Book RB024. See our guidance on standard techniques for generating, collecting, and testing gases.There are two methods given in the standard techniques for generating chlorine. The method that uses sodium chlorate(I) (sodium hypochlorite) is safer than the method that uses potassium manganate(VII), but will not work well if the sodium chlorate(I) (sodium hypochlorite) is an old sample. Note that sodium chlorate(I), NaOCl, is NOT the same as chlorate(V), NaClO3.
- Sodium chlorate(I) solution, 10–14% (w/v) NaOCl, fresh (CORROSIVE) – see CLEAPSS Hazcard HC089.
- Hydrochloric acid 5 M, HCl(aq) – see CLEAPSS Hazcard HC047a.
Procedure
Chlorine
- Place a 1 g tuft of cleaned iron wool in the reduction tube so that it is well spread out. Leave at least a 1 cm gap between the stopper and the iron wool.
- Connect the reduction tube to the chlorine generator with a short length of rubber tubing. Clamp it in position over a Bunsen burner.
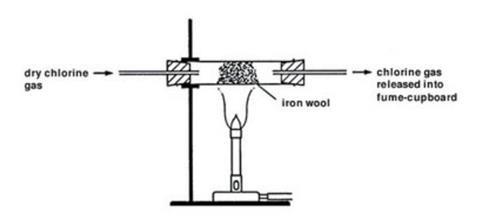
- Pass a slow stream of chlorine over the iron wool from the chlorine gas generator. Do this by allowing the hydrochloric acid to drip slowly on to the potassium manganate(VII). After a few seconds, it should be possible to see the greenish colour of the chlorine gas filling the reduction tube, as all the air is expelled.
- The iron wool may ignite without any heating. If not, gently heat at the end nearest to the chlorine generator until the wool does ignite (no further heating should be required).
- A vigorous reaction will occur and the glow will spread along the wool in the tube, producing clouds of brown iron(III) chloride. Some of this may emerge as a smoke from the end of the reduction tube.
- Continue passing chlorine over the iron wool until no further reaction occurs. Stop the chlorine supply and allow the tube to cool.
- When cool, disconnect the reduction tube and rinse a little of the product into a clean beaker with some distilled water. Pour some of this solution into a clean test tube and test with a few drops of silver nitrate solution. A white precipitate of silver chloride will form, confirming the presence of chloride ions.
Bromine
- Wear suitable protective gloves and take care to avoid spillage when handling liquid bromine. It is CORROSIVE and VERY TOXIC. Transfer about 0.5 cm3 liquid bromine into one of the boiling tubes, using the teat pipette. Care is needed to avoid spillage – the density and volatility of the bromine cause it to drip very easily from the pipette. Keep the bromine container and the mouth of the test tube close together. Replace the lid of the bromine container immediately.
- Using tongs or tweezers, place a 1 g tuft of cleaned iron wool into the boiling-tube so that it is well spread out and almost fills the boiling-tube. Leave a 2 cm gap between the iron wool and the surface of the liquid bromine.
- Clamp the test tube near the top and at an angle of about 45° – see diagram.
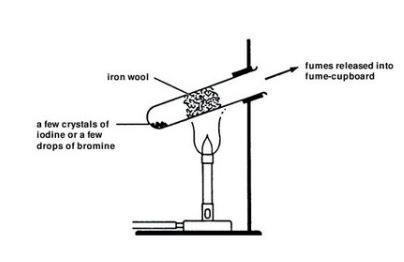
- Heat the test tube, gently at first, with a yellow-tipped blue flame (air hole on Bunsen burner slightly closed). Do this by moving the flame slowly between the bottom half of the iron wool and the bromine. As the bromine vapour starts to rise up into the iron wool, heat the wool more strongly.
- Remove the heat when the wool starts to glow due to the heat of the reaction. Note the extra heating required to get this reaction started compared to the reaction involving chlorine. The iron will become coated with yellow-brown iron(III) bromide, and a brown ‘smoke’ may escape from the mouth of the test tube.
- When the reaction appears to be over, use tongs or tweezers to remove some of the remaining iron wool from the test tube.
- Rinse the iron wool in a few cubic centimetres of deionised/distilled water in a small beaker. Pour out some of the resulting solution into a clean test tube and test with a few drops of silver nitrate solution. Formation of a cream precipitate of silver bromide confirms that bromide ions are present.
Iodine
- Transfer about 0.5 g of solid iodine (HARMFUL) into one of the boiling tubes. Place a 1 g tuft of cleaned iron wool in the test tube and clamp it as before.
- Working in a fume cupboard, heat the test tube with a yellow-tipped blue flame (air hole on Bunsen burner slightly closed). Heat gently at first by moving the flame slowly between the bottom half of the iron wool and the iodine.
- As the purple iodine vapour starts to rise up into the iron wool, heat the wool more strongly. Remove the heat when the reaction causes a dull glow – see Teaching notes below. Some red-brown iron(III) iodide should form.
- When the reaction appears to be over, remove some of the remaining iron wool from the test tube with tweezers and rinse it in a few cubic centimetres of deionised/distilled water in a small beaker.
- Pour some of the resulting solution into a clean test tube and test it with a few drops of silver nitrate solution. Formation of a yellow precipitate of silver iodide confirms that iodide ions are present.
Teaching notes
The order in which the experiments are done is a matter of choice, but it is probably best to leave the most reactive halogen (chlorine) to last, to end with a vigorous reaction – and confirm a class prediction?
The reaction with iodine is much less vigorous than that with bromine and it may be difficult to see a glow at all. A couple of trial experiments beforehand may be necessary to get the right balance between heating the iodine and getting the iron hot enough for a reaction to start. If the iron is heated too vigorously, it may start to glow from reaction with the oxygen in any air that may still be present in the test tube.
The general equation for the reactions involved is:
2Fe(s) + 3X2(g) → 2FeX3(s) (X = Cl, Br and I)
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet