Use this demonstration or class practical to illustrate changes to equilibria in carbonated soda water
In this experiment, students place soda water in a syringe. As they pull the plunger out to reduce the pressure above it, they can observe bubbles of carbon dioxide forming (‘out-gassing’) as its solubility decreases. Methyl red indicator added to the soda water turns from red to yellow, showing that the solution has become less acidic as the equilibria in solution adjust.
This short experiment can be carried out as a demonstration or as a class practical, with students working in pairs. They should be familiar with the phenomenon of out-gassing when carbonated drinks are opened. Students may not be familiar with methyl red indicator. If so, demonstrate its colours in acidic and alkaline solutions beforehand. It is red below pH 4.2 and yellow above pH 6.3.
Time needed is 5–10 minutes.
Equipment
Apparatus
- Eye protection
- Plastic syringe, 50 cm3 (modified – see note 5 below)
- Syringe cap (optional)
- Nail, 5 cm
- Beaker, 100 cm3
Chemicals
- Soda water or carbonated mineral water, a few cm3
- Methyl red indicator solution, a few drops
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Soda water or carbonated mineral water – a fresh, unopened bottle is best. Flavoured fizzy drinks, such as lemonade or cola, are not suitable here because they contain added acids, such as citric acid.
- Methyl red indicator solution – see CLEAPSS Hazcard HC032 and CLEAPSS Recipe Book RB000.
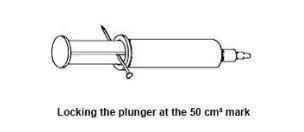
- A smaller syringe will do but the changes are less easily visible. Modify the syringe as follows: pull out the plunger so that the volume of air in the syringe is 50 cm3 (see diagram). Warm the nail in a Bunsen flame and push it through the stem of the plunger as shown in the diagram. When the nail is in place, the plunger can be ‘locked’ at the 50 cm3 mark.
Procedure
- Pour 10–20 cm3 of soda water into the beaker and add a few drops of methyl red indicator to give a red solution.
- Remove the nail from the syringe and insert the plunger completely. Draw about 5 cm3 of the soda water and indicator solution into the syringe. Place a syringe cap over the end of the syringe (or use a finger), pull the plunger out to the 50 cm3 mark and lock it with the nail. Bubbles of carbon dioxide will be seen out-gassing from the water and the indicator will begin to turn orange. Shake the syringe to speed up the out-gassing.
- Hold the syringe vertically with the nozzle pointing upwards, remove the syringe cap and the nail, and push in the plunger to expel the gas but not the solution. Seal the syringe again and repeat the out-gassing cycle in step 2. More bubbles will be seen and the indicator will turn further towards a yellow colour. Several more such cycles can be repeated until the indicator becomes completely yellow.
Teaching notes
A white background helps. Place the syringe next to the original red solution to emphasise the colour change.
An additional demonstration that shows the effect of temperature on the solubility of a gas, and the associated indicator colour changes, involves boiling some soda water containing a little methyl red indicator in a boiling tube. This will expel the carbon dioxide, which is less soluble at high temperatures, and shows the colour change of the indicator from red to yellow.
Soda water contains carbon dioxide that has been dissolved in it under pressure. The equilibria involved in this experiment are:
- CO2(g) ⇌ CO2(aq)
- CO2(aq) + H2O(l) ⇌ H2CO3(aq) (carbonic acid)
- H2CO3(aq) ⇌ H+(aq) + HCO3–(aq) (hydrogencarbonate ions)
- HCO3–(aq) ⇌ H+(aq) + CO32–(aq) (carbonate ions)
(For simplicity, teachers may prefer not to discuss this last equilibrium).
The solution of carbon dioxide is thus acidic because of the increase in concentration of H+(aq) ions resulting from these reactions. Reducing the pressure causes CO2 to come out of solution, ie equilibrium 1 moves to the left. The result is that the other three equilibria also move to the left, removing H+(aq) ions from the solution and making the solution less acid.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.


















No comments yet