Try this class experiment to investigate how much energy different foods contain
In this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food.
This is a class experiment in which different groups can investigate different foodstuffs. If each group investigates two foodstuffs – one in common with the rest of class to provide a common baseline, and the other a different foodstuff from the rest – a comparative table of energy in different foods can be drawn up from the class results. This should be possible to achieve in 45–60 minutes.
Equipment
Apparatus
- Eye protection
- Thermometer (–10 to 110 °C), short, stirring type
- Boiling tube, or metal calorimeter (or similar metal container) (see note 4 below)
- Measuring cylinder, 25 cm3
- Bunsen burner
- Heat resistant mat
- Mounted needle
- Teaspoon
- Stand and clamp
- Balance, weighing to 0.1 g
Chemicals
Use of a variety of dry foodstuffs, such as:
- Mini-marshmallows
- Popcorn (already popped)
- Crisps
- Pasta
- Bread
- Potato
- Bacon
- Broad beans (dried)
- Cheese
See notes 5, 6 and 7 below for additional guidance.
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Students must be instructed NOT to taste or eat any of the foods used in the experiment.
- While boiling tubes are easy to use for this experiment, the poor thermal conductivity of glass may be a major cause of error. Metal containers, such as copper calorimeters or tin cans of similar dimensions that can be held in a clamp, provide more effective heat transfer to the water. Note that this benefit is lost if the can is stood on a tripod, as the latter will also be heated.
- Check in advance for common allergy problems, eg peanuts.
- Some foodstuffs can be burned safely and easily using a mounted needle. Others may melt and drop off the needle, so burning on an old metal teaspoon is an alternative method – this can also be used for liquid foodstuffs, such as olive oil. High protein foodstuffs may produce pungent fumes, and should be burned in a fume cupboard. Each foodstuff provided should be tested beforehand to check that it is capable of sustained combustion without having to be relit repeatedly.
- One foodstuff needs to be selected as suitable for the standard experiment used by each group. Enough samples of approximately the same mass of this foodstuff need to be provided for the class. A trial run before the lesson should be carried out to establish that it will burn readily, sustain combustion and leave little unburnt residue, and that the mass of this foodstuff that will cause a temperature rise in the water used of around 20–30 °C.
Procedure
Using a test tube
- Measure 10 cm3 of water into the test tube.
- Clamp the test tube in the retort stand at an angle as shown in the diagram, and over a heat resistant mat.
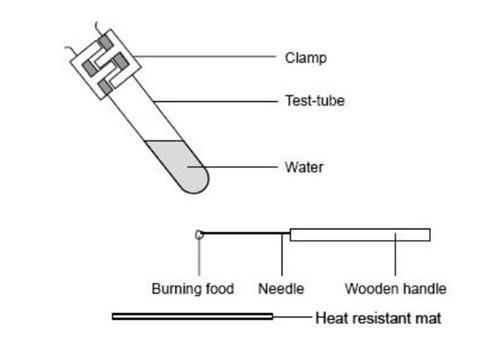
- Weigh a small piece of food and record the mass.
- Take the temperature of the water and record it in the table.
- Fix the food on the end of the mounted needle. If the food is likely to melt when heated put it on a teaspoon instead of on the needle.
- Ignite the food using a Bunsen burner, and immediately hold it about 1 cm below the test tube and above a heat resistant mat. If the flame goes out, quickly relight it.
- When the food stops burning, stir the water with the thermometer and record the temperature.
- If there is a significant amount of unburnt food left on the needle, reweigh this and record the mass remaining.
- Empty the test tube and refill it with another 10 cm3 of cold water. Repeat the experiment using a different food.
Using a metal container
Instructions as for a test tube, except:
- Use a larger volume of water, eg 25 or 50 cm3, and a larger food sample.
- Clamp the container in a level position above a heat resistant mat.
Teaching notes
This experiment provides an opportunity to use a temperature sensor linked to a data logger instead of a thermometer.
One foodstuff should be preselected to be the one used by all groups to standardise their experiments with each other (see Health, safety and technical notes), while each group needs to be allocated a different foodstuff for the second run.
As the same amount of water is heated each time, the temperature rise can be used to compare the amount of heat energy given off per gram of each foodstuff by dividing the rise by the mass of foodstuff burnt.
For classes familar with the equation q = m × C × ΔT, where q is the heat energy, m the mass of water heated, C the specific heat of water (4.2 J g–1 deg–1) and ΔT the temperature rise, this can be used to calculate the energy (in J) absorbed by the water each time. Dividing this by the mass of foodstuff burnt gives the heat energy absorbed by the water in J per g, as shown in the table below.
Each group needs to prepare a results table along the lines of the example below:
| Measurement | Food 1 | Food 2 |
|---|---|---|
| Mass of foodstuff /g | ||
| Temperature of water before heating / o C | ||
| Temperature of water after heating / o C | ||
| Change in temperature / o C | ||
| Heat absorbed by water / J (Mass of water × 4.2 × Temp. change) | ||
| Heat absorbed by water per gram of food / J |
Class results for the heat absorbed by water per gram of food may then be collected and compared on a class spreadsheet prepared by the teacher. After this has been done, a class discussion of ‘fair test’ problems will be appropriate, identifying sources of error and ideas for improving the technique used.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet