This models the industrial process of cracking larger hydrocarbons to produce smaller alkanes for petrol
This experiment is intended as a demonstration, but could with the most competent students be a class practical. The main risk to be considered in making this choice is the handling of very hot glassware and manipulating the apparatus for the safe collection of the flammable gas mixture over water.
As a class practical, it is best if the students work in pairs, with one student controlling the Bunsen burner and the other collecting the tubes of gas.
Students not familiar with using bromine water and potassium manganate(VII) solution to test for unsaturation need to be taught these tests first, using cyclohexane and cyclohexene.
The demonstration will take about 15–20 minutes, including testing of the gases. A class experiment should take about 45 minutes.
Equipment
Apparatus
- Eye protection (goggles)
- Safety screens
- Test tubes x4
- Bungs, to fit test tubes, x4
- Test tube rack
- Boiling tube (note 4)
- Bung, one-holed, to fit boiling tube
- Delivery tube fitted with a Bunsen valve (note 5)
- Small glass trough or plastic basin, for gas collection over water
- Bunsen burner
- Heat resistant mat
- Stand and clamp
- Dropping pipette
- Wooden splint
Chemicals
- Medicinal (liquid) paraffin (not the fuel), about 2 cm3
- Porous pot or pumice stone fragments (note 10)
- Bromine water, 0.01 M – diluted to a pale yellow-orange colour, (HARMFUL), about 2 cm3
- Acidified potassium manganate(VII) solution, about 0.002 M, about 2 cm3
- Mineral wool (preferably ‘Superwool’)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection.
- During the experiment the students and teacher should be protected by safety screens in case of unexpected suck-back causing the hot tube to shatter.
- The boiling tube should be a hard glass (borosilicate) 150 mm x 25 mm test tube.
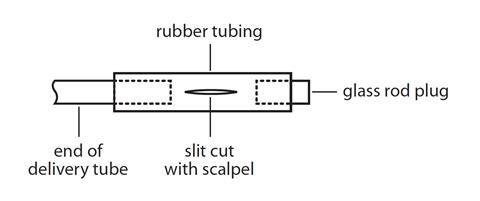
- It is important to ensure that the bung and the boiling tube fit well. Bunsen valves (see diagram below) can be made by attaching a 3 cm long piece of clean, unused, soft rubber tubing to the delivery tube, and then attaching a short length (1–2 cm) of glass rod, as shown in the diagram below. The rubber tubing should be slit on one side along about 1 cm of its length in the direction of the tubing. The use of a Bunsen valve should stop suck-back occurring. Safety screens must be used. See CLEAPSS Laboratory Handbook 13.2.1.
- Medicinal (liquid) paraffin (not the fuel) – see CLEAPSS Hazcard HC045b.
- Bromine water, Br2 (aq) (HARMFUL) – see CLEAPSS Hazcard HC015b and CLEAPSS Recipe Book RB017. Dilute the bromine water until it is pale yellow-orange in colour.
- Acidified potassium manganate(VII) (potassium permanganate) solution, KMnO4 (aq) – see CLEAPSS Hazcard HC081 and CLEAPSS Recipe Book RB073.
- Mineral wool (preferably ‘Superwool’) – see CLEAPSS Hazcard HC086A.
- Porous pot chips can be made by crushing broken crucibles into pea-sized fragments.
Procedure
- Place about a 2 cm3 depth of mineral wool in the bottom of the boiling tube and gently press it in place with a glass rod. Drop about 2 cm3 of liquid paraffin on to the wool, using a dropping pipette. Use enough paraffin to completely soak the mineral wool, but not so much that the paraffin runs along the side of the tube when it is placed horizontally.
- Clamp the boiling tube near the mouth so that it is tilted slightly upwards, as shown in the diagram below. Place a heap of catalyst (pumice stone or porous pot fragments) in centre of the tube and fit the delivery tube.
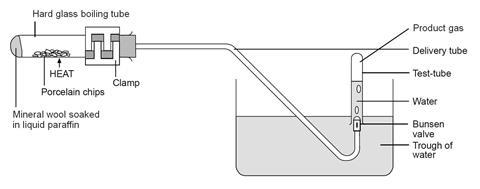
- Fill the trough about two-thirds full with water and position the apparatus so that the end of the delivery tube is well immersed in the water.
- Fill four test-tubes with water and stand them inverted in the trough. Also place the test tube bungs, upside down, in the water.
- Strongly heat the catalyst in the middle of the tube for a few minutes, until the glass is up to a dull red heat. Avoid heating the tube too close to the rubber bung.
- While keeping the catalyst hot, flick the flame from time to time to the end of the tube for a few seconds to vaporise some of the liquid paraffin. Try to produce a steady stream of bubbles from the delivery tube. Be careful not to heat the liquid paraffin too strongly or let the catalyst cool down. To avoid suck-back do not remove the flame from heating the tube while gas is being collected. If suck-back looks as if it is about to occur, lift the whole apparatus by lifting the clamp stand.
- When a steady stream of gas bubbles is established, collect four tubes full of gas by holding them over the Bunsen valve. Take care not to lift the water-filled tubes out of the water when moving them, to avoid letting air into them. Seal the full tubes by pressing them down on the bungs, then place them in a rack.
- When gas collection is complete, first remove the delivery tube from the water by tilting or lifting the clamp stand. Only then stop heating.
- Test the tubes of gas as follows:
- What does the gas look like? Carefully smell the contents of the first test tube. Of what does the smell remind you? Does liquid paraffin have a smell?
- Use a lighted splint to see if the gas is flammable. The first tube may contain mostly air. If it does not ignite, try the second tube. Once the gas is lit, invert the test tube to allow the heavier-than-air gas to flow out and burn.
- To the third tube of gas add 2–3 drops of bromine water, then stopper and shake well.
- To the fourth tube add 2–3 drops of acidified potassium manganate(VII) solution, then stopper and shake well.
Teaching notes
The demand for petrol is greater than the gasoline fraction obtained by distilling crude oil. Cracking larger hydrocarbons produces smaller alkanes that can be converted into petrol. It also produces small alkenes, which are used make many other useful organic chemicals (petrochemicals), especially plastics. This experiment models the industrial cracking process.
You need to be particularly vigilant and lift the apparatus out of the water if suck-back starts to occur and cannot be reversed by stronger heating .
It is very important to stress that students should not stop heating the boiling tube at the end of the experiment until the delivery tube is out of the water and contains no water.
The gas mixture which collects has a characteristic smell, burns with a yellow flame, and decolourises bromine water and acidified potassium manganate(VII) solution. This shows the presence of unsaturated molecules.
Students will find it helpful to build molecular models to understand the reaction and be able to write an equation for the reaction.
Additional information
Few websites deal with this topic at the 14–18 chemistry teaching and learning level – most are either far too technical or too elementary. However, Chemguide features a discussion of the cracking of hydrocarbons for A-level chemistry students and teachers.
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry

















No comments yet