Use this practical or demonstration to produce polystyrene as an example of addition polymerisation
Alkenes (carbon compounds containing carbon–carbon double bonds) undergo addition reactions. In this experiment, students observe the reaction that takes place as molecules of phenylethene or styrene – the monomer – add on to each other to form the polymer polyphenylethene, commonly known as polystyrene. This process is started by adding a substance called a free-radical initiator.
Because examples of addition polymerisation are difficult to demonstrate, or for students to experience themselves, this experiment is important either as a teacher demonstration or as a class experiment. The latter is only suitable for particularly able and reliable students, given the hazards of the substances involved, and the careful handling required.
The experiment takes up to 60 minutes, and should not be left unattended. However, it should be possible to make use of some of the ‘watch and wait’ time for theoretical work, for example on addition polymerisation and addition polymers.
Equipment
Apparatus
- Eye protection
- Disposable nitrile gloves
- Access to a fume cupboard
- Boiling tube, 150 x 25 mm
- Bung, one-holed, fitted with a 20 cm length of glass tubing (see the diagram below)
- Beakers, 100 cm3 and 250 cm3 (one of each)
- Glass stirring rod
- Stand and clamp
- One of the following (see note 8 below):
- Electric hotplate with thermostatic control
- Bunsen burner, tripod, gauze and heat resistant mat
Chemicals
- Phenylethene (styrene) (HARMFUL, FLAMMABLE), 5 cm3 (see notes 3, 4 and 5 below)
- Di(dodecanoyl)peroxide (lauroyl peroxide) (OXIDISING), 0.1 g
- Ethanol (IDA – industrial denatured alcohol) (HIGHLY FLAMMABLE, HARMFUL), 50 cm3 (see notes 7 and 9)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout, and use disposable nitrile gloves, particularly during the pre-treatment of phenylethene.
- Phenylethene vapour is narcotic in high concentrations, so this experiment must be carried out in a fume cupboard.
- Phenylethene (styrene), C8 H8 (l), (HARMFUL, FLAMMABLE) – see CLEAPSS Hazcard HC071. Check that the stock of phenylethene is in good condition. In spite of the presence of an inhibitor, phenylethene in store will gradually polymerise and eventually turn solid in the bottle.
- For the pre-treatment of phenylethene (CAUTION: wear goggles and chemical resistant gloves for this procedure): most phenylethene samples contain 4-(dimethylethyl)-benzene-1,2-diol, (4-tert-butyl catechol) (HARMFUL) as an inhibitor. This needs to be removed by washing with 1 M sodium hydroxide solution, NaOH(aq), (CORROSIVE), then with water, in a separating funnel. The phenylethene then needs to be dried over anhydrous sodium sulfate, Na2 SO4 (s), for 10 minutes. Wash all the apparatus used in propanone, CH3 COCH3 (l), (HIGHLY FLAMMABLE, IRRITANT) as soon as possible.
- Di(dodecanoyl)peroxide (lauroyl peroxide), (CH3 (CH2)10 CO)2 O2 (s), (OXIDISING) – see CLEAPSS Hazcard HC029.
- Ethanol (IDA – industrial denatured alcohol), C2 H5 OH(l), (HIGHLY FLAMMABLE, HARMFUL) – see CLEAPSS Hazcard HC040A.
- The boiling water bath required can be set up more quickly using boiling water from an electric kettle, and kept boiling on a thermostatically-controlled electric hotplate. This is a safer alternative to the use of a Bunsen burner in this experiment.
- Provide a chemical waste collection container so that the ethanol washings can be collected and disposed of safely after the lesson. The polystyrene samples can be disposed of as normal waste.
Procedure
- Prepare a 250 cm3 beaker of boiling water to act as a water bath. If using a Bunsen burner, keep all other chemicals well away from the flame.
- Add 0.1 g of di(dodecanoyl) peroxide to 5 cm3 of phenylethene in a boiling tube.
- Fit a bung carrying a 20 cm length of glass tubing in the top of the boiling tube. This minimises the escape of phenylethene vapour. Clamp the tube vertically in the boiling water bath so that the liquid in it is below the level of the hot water – see the diagram below.
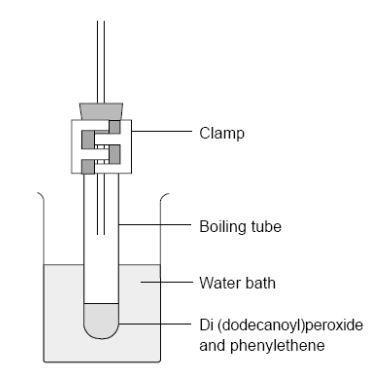
- Heat for about 30 min until the liquid turns quite viscous, remove from the water bath and leave to cool.
- Extinguish all flames. Pour the contents of the tube into 50 cm3 of ethanol in a beaker.
- Use a glass rod to push the poly(phenylethene) into a lump and pour off the ethanol.
- Dry the solid polymer on a filter paper.
Teaching notes
Note
With the use of a powerful oxidising agent, and also flammable and harmful substances, there is need for eye protection, disposable gloves and a fume cupboard.
Students will need some theoretical background to addition reactions of unsaturated compounds and to polymerisation before this experiment. The reaction in this experiment is:
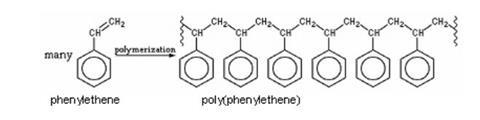
Poly(phenylethene) is of course better known as polystyrene. However, many students will relate the name ‘polystyrene’ to the foamed material, expanded polystyrene, and be less familiar with the glassy solid. Teachers may wish to have samples of objects made of solid polystyrene to show students. Possibilities include yoghurt pots, margarine tubs, clear egg boxes, food packaging trays, plastic cutlery and cups, clear plastic glasses, ball-point pen cases, CD cases and plastic coat hangers.
Look at the recycling code on such objects. Note that many of these also contain fillers, plasticisers, pigments and other components.
The brittle, glassy nature of pure polystyrene (consider a CD case) is due to the bulky benzene rings projecting from the side of the carbon chain, which limit the flexibility of the chain. However, it is also a remarkably strong material, leading to a very wide variety of applications.
The initiator, di(dodecanoyl)peroxide, has a peroxy group, –O–O–, in the middle of the molecule, between two large dodecanoyl, CH3 (CH2)10 CO–, groups. This molecule splits readily at the O–O bond, leaving unpaired electrons on each of the oxygen atoms, resulting in the formation of two free radicals, CH3 (CH2)10 COO.. It is these free radicals that attack and open the double bond in phenylethene, propagating a sequence of unpaired electrons at the end of the growing carbon chains. This is why only a tiny amount of initiator is needed to set off the reaction.
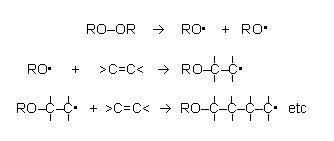
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry

















No comments yet